Rheumatoid arthritis (RA) is among the most common autoimmune diseases, affecting roughly 1% of the world's population (1). The disease is progressive in nature, typically beginning with swollen and tender joints of the hands/feet and eventually progressing to larger joints, joint degradation, and severely limited mobility if left untreated. The most common treatment today is methotrexate, but newer technology has allowed for specific targeting of cytokines implicated in disease pathology (i.e. TNF-α) to slow the progression. Even with recent technological advancements, it is still extremely difficult to definitively determine the cause of any individual case of RA. Part of this difficulty can be attributed to the current lack of an animal model that mimics the complex nature of human RA. However, a variety of rat and mouse models have been developed over the years to tease out the contributions of immune, genetic, and environmental factors to RA in order to develop more efficacious treatments (2,3).
Recent research (4-6) has begun to elucidate the role that environmental factors, namely bacteria and bacterial toxins constituting human microbiota, have in autoimmune disease pathogenesis. The human gastrointestinal tract harbors a staggering array of bacterial species that have been shown to play a pivotal role in stimulating and educating our immune systems (7), and therefore our susceptibilities to autoimmune diseases like RA. Lipopolysaccharide (LPS), a unique outer-membrane component of Gram-negative bacteria such as E. coli, is of particular interest since it has been implicated in rheumatoid arthritis. LPS has been shown to activate Toll-like receptor 4 (TLR-4) (8), which in turn stimulates the production of pro-inflammatory cytokines.
Determining how human microbiota contributes to autoimmune disease pathogenesis could lead the way to more effective treatments, and perhaps even prophylactic measures, for RA. To do so, it is vital to establish animal models that are capable of discerning the effect that bacteria and bacterial toxins have on the initiation and progression of RA. At Chondrex, we provide three mouse models that can be adapted to study the effects of bacterial components, such as LPS, SEB, MAM, PG-PS and others, on the development of RA.
1. Collagen Antibody-Induced Arthritis
Collagen Antibody-Induced Arthritis (CAIA) is the fastest, most consistent murine arthritis model. The CAIA model bypasses the antigen recognition and activation phases of the immune response by injecting a 5-clone cocktail of monoclonal antibodies to various conserved epitopes in type II collagen. Since the traditional CIA model (discussed below) requires a restricted MHC haplotype, arthritis can only be induced in particular mouse strains. The CAIA model does not require B-cell or T-cell activity, meaning that arthritis can be induced in virtually any mouse strain within 24-48 hours. This flexibility permits the usage of mice with a wide range of genetic backgrounds, which is useful to elucidate the interplay between genetic and environmental components of RA etiology.
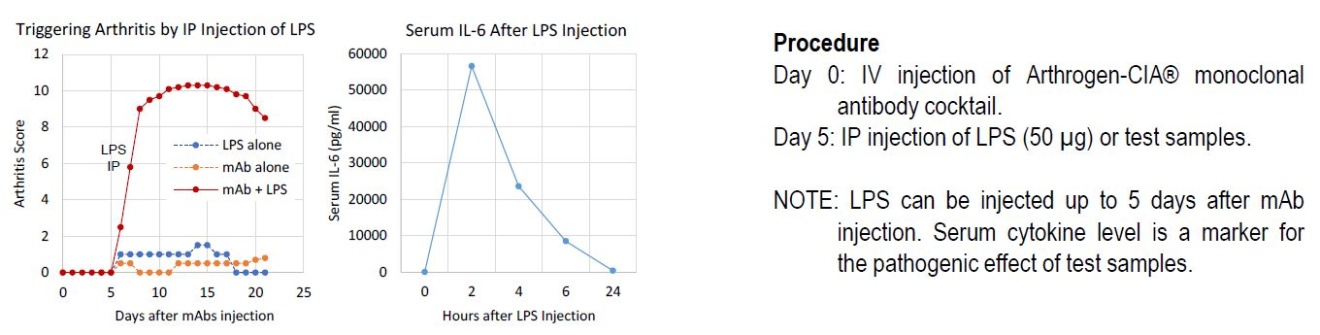
At high enough doses, Arthrogen-CIA® monoclonal antibody (mAb) cocktail can induce arthritis in mice by itself. More interestingly, however, is that when mice receive a sub-arthritogenic dose of Arthrogen-CIA® mAb cocktail followed by IP injection of LPS (50 μg), there is a drastic increase in arthritis severity as compared to LPS or Arthrogen-CIA® alone (Figure 1). While the exact mechanism behind this phenomenon is not entirely clear, it is apparent that LPS can play a role in RA pathogenesis by stimulating the production of pro-inflammatory cytokines.
Figure 1
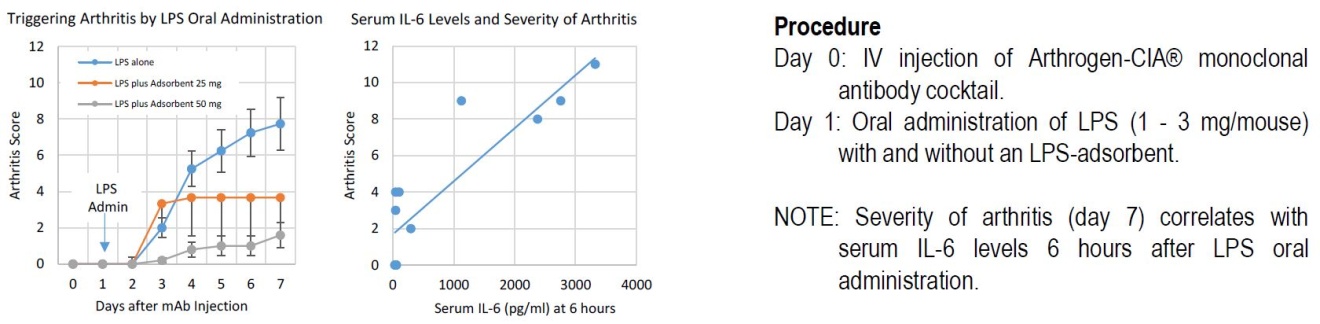
In addition, oral administration of LPS can be used in the CAIA model. This protocol more closely resembles the exposure to LPS that a RA patient would experience in everyday life. Figure 2 demonstrates how oral administration of LPS, in conjunction with IV injections of Arthrogen-CIA® mAb cocktail, affects the severity of arthritis in a dose dependent manner. Figure 2 also shows that serum interleukin-6 (IL-6) levels 6 hours after LPS feeding correlate with arthritis severity. IL-6 is a known pro-inflammatory cytokine, thus reinforcing the idea that LPS translocation from the gastrointestinal tract leads to stimulation of the inflammation pathway.
Figure 2
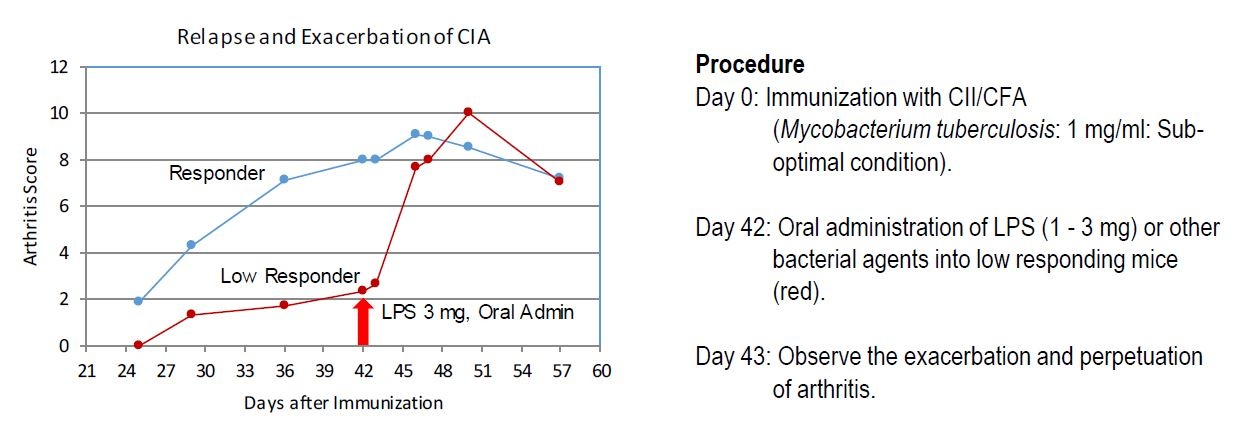
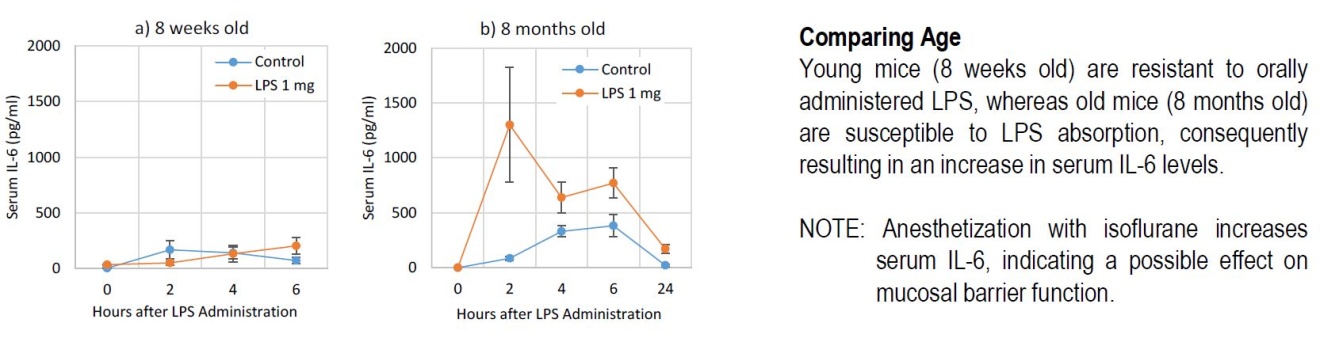
Furthermore, when the Arthrogen-CIA® mAb cocktail was administered to young mice (8 weeks) and old mice (8 months) using the LPS oral feeding protocol, old mice exhibited increased serum IL-6 levels (when compared to controls) while young mice did not (Figure 3). This age discrepancy suggests that changes in gut mucosal membrane permeability associated with aging (immunosenescence) affects LPS translocation in vivo. These findings mirror the age bias seen in human RA patients, with most patients affected being of an advanced age.
Figure 3
2. Collagen-Induced Arthritis
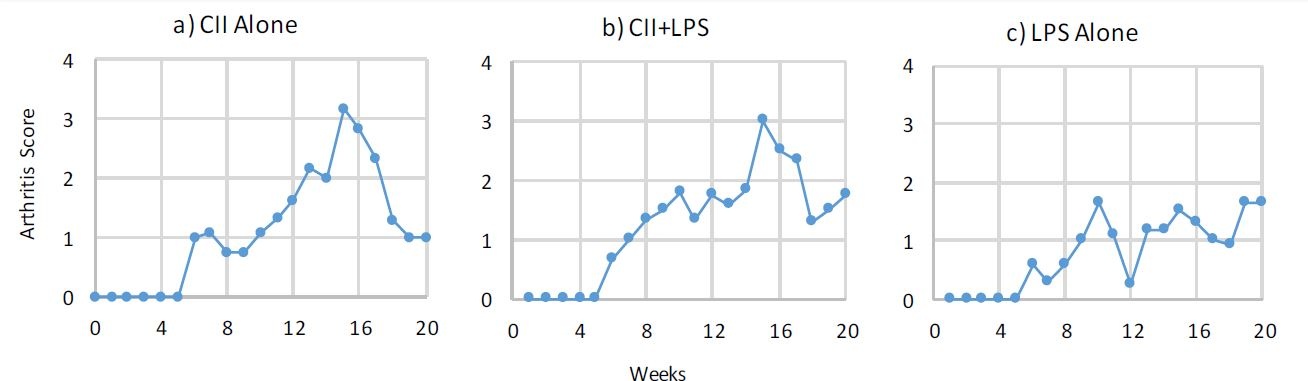
Collagen-Induced Arthritis (CIA) is one of the most popular animal models of RA. Traditionally, mice are immunized with heterologous type II collagen (usually bovine) and Complete Freund's Adjuvant (CFA) that acts as an immunopotentiator. Just as in the CAIA model, bacterial toxins (i.e. LPS) can evoke inflammation in the CIA model by increasing the production of pro-inflammatory cytokines (Figure 4).
Figure 4
Susceptibility to the CIA model depends on MHC haplotypes, therefore only certain mouse strains can be used (DBA/1, B10.RIII, etc.). This model is ideal for studying the role of T-cells and B-cells in RA pathogenesis and can be adapted to study the synergistic effects of any intestinal bacteria/toxin in RA development.
3. Oral Collagen-Induced Arthritis
Under the mimic antigen hypothesis, it is thought that ingesting heterologous type II collagen elicits antibodies that cross-react to conserved epitopes of human type II collagen and induce an autoimmune response. The Oral Collagen-Induced Arthritis (OCIA) model most closely resembles this hypothesis of RA development in humans. Oral feeding of mimic antigen (heterologous type II collagen) and LPS has been shown to induce mild, but chronic arthritis (Figure 5). This model is useful for studying the long-term impact that mimic antigens and/or bacterial components, in conjunction with the host's immune function, have on RA pathogenesis.
Figure 5
The hygiene hypothesis posits that the increase in the incidence of autoimmune diseases in developed nations can be attributed, at least in part, to the decrease in the microbial exposure people experience within those nations. From an evolutionary standpoint, this hypothesis makes perfect sense. Animals and bacteria have co-evolved over hundreds of millions of years to form both the symbiotic and parasitic relationships they share today, but within the last 200 years, new selective forces have been exerted on human microbiota. Whether it is improved hygiene, increased sterility measures, increases in living standards, or the prevalence of antibiotic usage, these factors have the ability to quickly and substantial change the composition of the human microbiota from what is was pre-industrial revolution. These compositional changes upset the normal balance of pro-inflammatory and anti-inflammatory cytokines, leading to the local and systemic inflammation common to autoimmune diseases. Elucidating the role that human microbiota composition has on immune system health is pivotal in expanding the current understanding of autoimmune diseases. The animal models briefly reviewed in here are essential research tools that can pave the way for the next generation of treatments for rheumatoid arthritis and other autoimmune diseases.
References
- Y. Alamanos, A. Drosos, Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 4(3), 130-6 (2005).
- D. Asquith, A. Miller, I. McInnes, F. Liew, Animal models of rheumatoid arthritis. Eur J Immunol 39(8), 2040-4 (2009).
- K. Kannan, R. Ortmann, D. Kimpel, Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 12(3), 167-81 (2005).
- T. Vatanen, A. Kostic, E. d'Hennezel, H. Siljander, E. Franzosa, M. Yassour, et al., Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 165(4), 842-53 (2016).
- X. Liu, B. Zeng, J. Zhang, et al. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci Rep 6, 30594 (2016).
- I. Ivanov, K. Atarashi, N. Manel, E. Brodie, T. Shima, U. Karaoz, et al., Induction of intestinal Th17 Cells by segmented filamentous bacteria. Cell 139(3), 485-98 (2009).
- J. Round, S. Mazmanian, The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9(5), 313-23 (2009).
- G. Kiyeko, E. Hatterer, S. Herren, C. Di, L. van, W. Reith, et al., Spatiotemporal expression of endogenous TLR4 ligands leads to inflammation and bone erosion in mouse collagen-induced arthritis. Eur J Immunol 46(11), 2629-2638 (2016).
Keywords: Rheumatoid Arthritis, Microbiota, Autoimmune Disease, Collagen Antibody-Induced Arthritis, Collagen Induced Arthritis, Rheumatoid Arthritis Model