The immune response against specific foreign antigens and pathogens is known as adaptive immunity. Adaptive immunity is mediated by lymphocytes, specifically T-cells and B-cells, that are responsible for cell-mediated immunity and humoral immunity, respectively. Individual T- and B-cells can only recognize a small structural section or peptide sequence of an antigen, known as their epitope. T-cells are activated when their T-cell receptors bind to an antigen-derived peptide (T-cell epitope) presented by major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs). B-cells are also activated when their B-cell receptors bind to a specific antigen presented by T-helper (Th) cells. Activated B-cells will then produce antibodies recognizing the peptide sequence or the structural conformation of the antigen (B-cell epitope). Therefore, T-cells and B-cells (antibodies) will respond to the specific epitope presented by APC or Th-cells, respectively, during the cell activation processes. The unique qualities of each epitope presented by APCs and Th-cells provides a variety of immune cell specificities against invading antigens, which helps in establishing a sufficient immune response against foreign antigens.
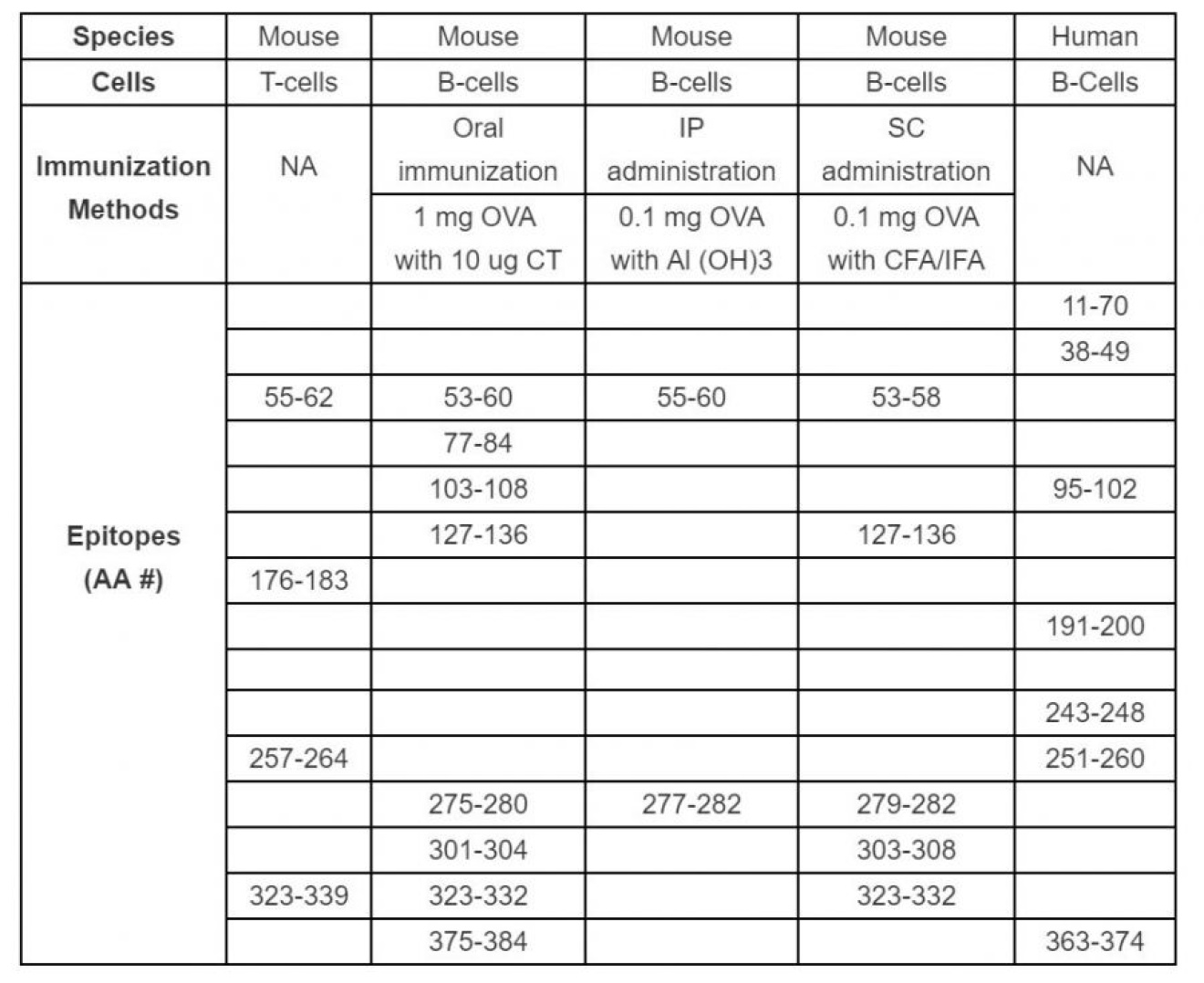
Ovalbumin (OVA) is used as a popular antigen in many allergic disease studies, primarily food allergy and allergic asthma models. Therefore, many researchers have reported T-cell and B-cell epitopes of OVA in the context of allergic immune responses. T-cell epitopes, such as OVA peptide of amino acid numbers 55-62 (OVA55-62), OVA176-183, and OVA257-264, have been identified to bind to MHC class I, H2-Kb receptors on T-cells in mice immunized with OVA. OVA257-264 elicited a strong T-cell response, while OVA176-183 elicited a low T-cell response. Interestingly, the binding of OVA55-62 to H2-Kb failed to elicit a cytolytic T-cell response (1). Furthermore, in another study, OVA323-339 induced either intestinal inflammation or tolerance depending on the MHC type of the mouse strain immunized with OVA (2) (Table 1). Thus, T-cell activation is highly dependent on the MHC type of subjects and specific peptide antigens.
On the other hand, anti-OVA antibodies in humoral immunity show a wider variety of epitopes. Epitopes of serum antibodies were identified by immunizing mice with OVA using several administration routes. Oral OVA immunization with Cholera toxin elicited IgE antibodies against a numerous epitopes (OVA53-60, 77-84, 103-108, 127-136, 275-280, 301-306, 323-332, and 375-384), while subcutaneous OVA immunization with Complete Freund's Adjuvant (CFA) elicited IgE antibodies against more limited epitopes (OVA53-58, 127-136, 275-280, 301-306, and 323-332) (3) (Table 1). Intraperitoneal OVA immunization with Aluminum Adjuvant (Alum) exhibited IgE antibodies against OVA55-60 and 227-282 (3). In another study, OVA immunization with Alum induced anti-OVA IgG antibodies recognizing epitope OVA323-339 (4), indicating that OVA323-339 may act as a common epitope for T-cells and B-cells in immune. When establishing animal models, especially allergic disease models, an appropriate immunization route and adjuvant must be used for evaluating humoral immunity against antigens.
Table 1. Summary of T-cell and B-cell (antibody) epitopes of OVA that have been identified in mice immunized with OVA peptides and humans with allergies to OVA. NA: Not applicable, AA#: Amino Acid Numbers of OVA peptide.
MHC types are species-specific, therefore different antibody epitopes were identified in human patients exhibiting allergies to egg white. OVA peptides were screened for their ability to induce mast cell activation using patient sera. Dominant IgE antibody epitopes (OVA 11-70, 38-49, 95-102, 191-200, 243-248, 251-260, and 363-374), were determined by activating basophil histamine release (Table 1) (5-7).
Chondrex, Inc provides several anti-OVA monoclonal antibodies (mAbs) with distinct epitopes determined by indirect ELISA using sequentially overlapping 12 amino acid long OVA peptide fragments ranging the entire OVA amino acid sequence (Peptide number 1-91) (Figure 1):
mAb clone E-C1 (Cat# 3006 and Cat# 7091): OVA61-68
mAb clone 2322 (Cat# 7094): OVA61-68
mAb clone 4B4E6 (Cat# 7096): OVA191-202
mAb clone E-G5 (Cat# 3007 and Cat# 7092): OVA191-202
mAb clone L71 (Cat# 3008 and Cat# 7093): OVA363-374.
Unfortunately, these mAbs do not recognize the mouse IgE epitopes previously published (Table 1). However, they do recognize epitopes similar to IgE epitopes in human patients and may work in studies mimicking human immunoreaction to allergens. Furthermore, two anti-OVA IgE mAbs, E-C1 and E-G5, recognize the same epitopes of anti-OVA IgG mAbs, 2322 and L71, respectively. These pairs of IgG and IgE mAbs may be useful for studying two roles of anti-OVA IgG antibodies in allergic responses: 1) Anti-OVA IgG antibodies compete with anti-OVA IgE antibodies on the same epitope on OVA, leading to lower mast cell activation, or 2) anti-OVA IgG antibodies collaborate with anti-OVA IgE antibodies to develop IgG-OVA immune-complexes, synergistically activating mast cells.
Additionally, Chondrex, Inc. determined that both mAb E-C1 administration, in conjunction with OVA sensitization, can induce mast cell activation and foot pad hypersensitivities in mice, but mAb E-G5 failed to do so. Mab E-G5 can be a useful negative control antibody in allergic research using OVA. Interestingly, the two IgE mAbs showed different band patterns on western blot analysis and reactivities in an antibody assay system (8). These different results may explain the roles of anti-allergen IgE antibodies in development of mouse disease models.
Figure 1. The OVA amino acid sequence (top) and epitope analysis of several Anti-OVA monoclonal antibodies (Clone E-C1, 2322, 4B4EG, E-G5, and L71) from Chondrex, Inc.
References
- G. Lipford, M. Hoffman, H. Wagner, K. Heeg, Primary in Vivo Responses to Ovalbumin. Probing the Predictive Value of the Kb Binding Motif. Journal of Immunology 150, 1212-1222, (1993).
- N. A. Haruyo et al., Two Distinct Epitopes on the Ovalbumin 323-339 Peptide Differentiating CD4+T Cells into the Th2 or Th1 Phenotype. Biosci Biotechnol Biochem 76, 1979-1981, (2014).
- Y. Mine, M. Yang, Epitope characterization of ovalbumin in BALB/c mice using different entry routes. Biochim Biophys Acta 1774, 200-212, (2007).
- L. Sun et al., Comparison Between Ovalbumin and Ovalbumin Peptide 323-339 Responses in Allergic Mice: Humoral and Cellular Aspects. Scandinavian journal of immunology 71, 329-335, (2010).
- S. Elsayed, L. Stavseng, Epitope Mapping of Region 11-70 of Ovalbumin (Gal D I) Using Five Synthetic Peptides. International archives of allergy and immunology 104, 65-71, (1994).
- K. Honma et al., Allergenic Epitopes of Ovalbumin (OVA) in Patients With Hen's Egg Allergy: Inhibition of Basophil Histamine Release by Haptenic Ovalbumin Peptide. Clinical and experimental immunology 103, 446-453, (1996).
- Y. Mine, P. Rupa, Fine Mapping and Structural Analysis of Immunodominant IgE Allergenic Epitopes in Chicken Egg Ovalbumin. Protein engineering 16, 747-752, (2003).
- Y. Okamoto-Uchida et al., Different Results of IgE Binding- and Crosslinking-Based Allergy Tests Caused by Allergen Immobilization. Biological & pharmaceutical bulletin 39, (2016).