Ovalbumin (OVA), the major protein component of avian egg-white, is one of the primary allergens (the other primary allergen being ovomucoid) for infants with egg white allergies. It is a 45 kDa glycoprotein that is part of the SERPIN (SERine Protease INhibitor) protein family, even though it does not exhibit inhibitor activity like other SERPINs (1). SERPINs are typically found in a metastable intermediate conformation with a flexible, exposed region of the protein (termed the reactive center loop or RCL) that acts as a substrate for serine proteases. When a serine protease binds to the RCL, it will cleave the loop at the P1 and P1' residues, allowing the serpin protein to undergo a conformation change from its intermediate state (with the RCL exposed) to its most stable conformation (Figure 1). This final conformation has the C-terminal end of the reactive center loop inserted inside the tertiary structure of the SERPIN, where it is incorporated into a β-sheet that helps stabilize the protein. If the RCL insertion occurs efficiently, the N-terminal of the RCL will remains bound to the protease through an ester bond inactivates the protease by destabilizing it with energy generated from the conformation change (1,2). If the RCL insertion does not happen fully or efficiently, the SERPIN will not covalently attach to the protease, allowing the active protease to disassociate. OVAs tertiary structure does contain the reactive center loop, but cleavage by a protease does not induce the incorporation of the reactive center loop into a β-sheet, a crucial step in protease inhibition by serpins. Therefore, despite sharing 30% of its sequence with functional serpin inhibitors, OVA lacks the ability to inhibit proteases (1). Although the biological function of OVA is not entirely certain, recent studies have attributed the protein to an essential role in embryonic development. The ongoing hypothesis is that OVA can serve as an amino acid source for the developing chicken embryo and plays a crucial role in calcium carbonate formation for the eggshell mineralization (3).
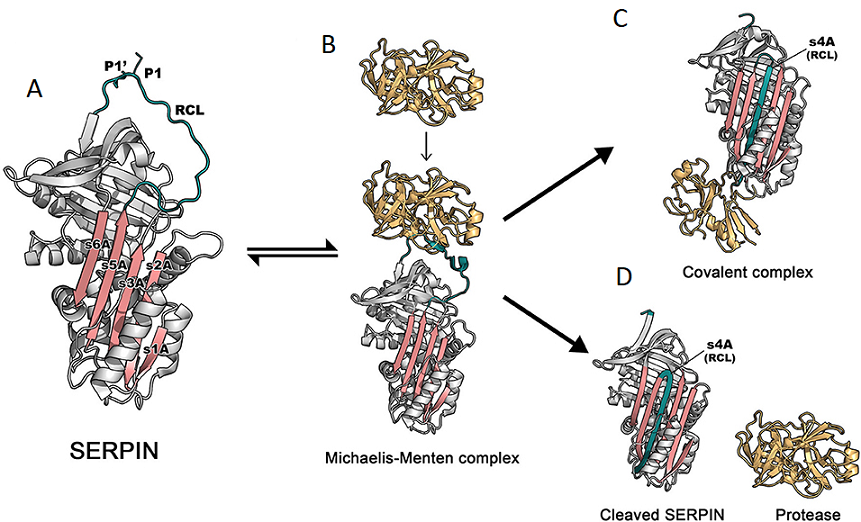
Figure 1. (A) The structure of the archetypal SERPIN, α1-antitrypsin. The reactive center loop (RCL) is in green, containing a protease cleavage site (P1-P1'). β-sheet A, comprising of 5 strands (s1A, s2A, s3A, s5A, and s6A) is in pink. These two regions serve as main features, which play an important role in the dramatic conformational change that SERPINs undergo during inhibition [PDB code: 1QLP]. (B) Initially, a target protease docks and binds the recognition site, exposed on the RCL. This step leads to formation of the non-covalent Michaelis-Menten complex [PDB code: 1OPH]. (C) Upon cleavage at P1-P1', the SERPIN spontaneously refolds into a hyperstable conformation, where the N-terminal portion of cleaved RCL is inserted between central β-sheet A. This conformational change of the SERPIN results in “trapping” the covalently linked protease into an inactive form [PDB code: 1EZX]. This SERPIN-protease complex will subsequently be eliminated from the circulation. (D) In some cases, a SERPIN can act as a substrate, where protease and SERPIN do not remain covalently linked, as is the case with OVA. When the RCL of OVA is cleaved, the insertion of the N-terminal into β-sheet A is insufficient, preventing the formation of an ester bond between the C-terminal of the RCL. This results in an active protease that disassociates from the SERPIN, which leaves the SERPIN in a cleaved form [PDB code: cleaved 7API].
*Adapted, with permission, from Sanrattana W, Maas C, de Maat S, SERPINs-from trap to treatment. Front Med (Lausanne) 6, 25 (2019).
While the biological function of OVA remains equivocal, OVA has found many applications in a wide range of biological research fields. Given its distinction as one of the first proteins purified in its crystalline form (4), and its wide availability, OVA was used extensively in early immunological studies (5-9). These studies helped lay the groundwork for our current understanding of antibody-protein interactions and antibody specificity. The historical use of OVA as an experimental antigen has persisted to today. As the second most common food allergen in infants, it is logical that OVA is used as an antigen to induce IgE-mediated food allergy animal models (10). The high allergenicity of OVA in mammals has allowed OVA to be used as an allergen to induce other IgE-mediated disease models such as the OVA-induced allergic asthma model (11) and atopic dermatitis models (12). It is important to note that using OVA containing low endotoxin levels is essential for allergy mode induction. Endotoxin contamination in OVA preparations will activate Th1 immune responses, rather than the desired Th2 immune responses that lead to the production of allergen specific IgE antibodies.
Beyond allergy research, OVA has been utilized as a model antigen to evaluate experimental vaccine delivery systems as. Indeed, the commercial availability of purified OVA, as well as Mouse Anti-OVA Antibody Assay Kits for quantifying OVA specific antibody subtypes/subclasses, makes OVA a very convenient and cost-effective model antigen for vaccine research. For instance, Korupalli et al. encapsulated OVA in an injectable nanocomposite hydrogel and were able to show that the hydrogel platform promoted a strong and prolonged immune response. By using Chondrex, Inc.'s Mouse Anti-OVA IgG Antibody Assay Kit, they could measure changes in anti-OVA antibodies after vaccine administration to monitor the prolonged immune stimulation by OVA (13). Recently, Dölen et al. used a similar method to study the efficacy of a cancer vaccine using nanoparticles, where a tumor antigen and an agonist for invariant natural killer T (iNKT) cells are co-delivered to induce strong anti-tumor T cell responses. To determine the best administration route of the vaccine, OVA was used (instead of a tumor antigen) to provide a convenient method to evaluate B-cell responses. Anti-OVA antibody levels (IgM, IgG1, IgG2c, and IgE) determined by ELISA showed how different administration routes affect the immune response to the vaccine (14). Furthermore, transgenic melanoma cell lines that express ovalbumin antigens (a pseudo-tumor antigens) provides a convenient way to study cancer vaccines (15).
Clearly, OVA has a plethora of ways it can be used in biological research. Whether it used as an allergic antigen for IgE-mediated disease induction, a model antigen to study vaccines, or as a tumor antigen in transgenic cancer cell lines, OVA is an ideal antigen for a variety of biological studies. For this purpose, Chondrex, Inc. provides purified OVA, as well as Anti-OVA Antibody Assay Kits, that are ideal for studies monitoring immune responses to OVA.
References
- J. Huntington, P. Stein. Structure and properties of ovalbumin. J Chromatogr B Biomed Sci Appl 756, 189-198 (2001).
- W. Sanrattana, C. Maas, S. de Maat, SERPINs-from trap to treatment. Front Med (Lausanne) 6, 25 (2019).
- C. Dombre et al., Egg serpins: The chicken and/or the egg dilemma. Semin Cell Dev Biol 62, 120-132 (2017).
- F. Hopkins, On the separation of a pure albumin from egg-white. J Physiol 25, 306-330 (1900).
- A. Tiselius, E. Kabat, An electrophoretic study of immune sera and purified antibody preparations. J Exp Med 69, 119-131 (1939).
- M. Heidelberger, K. Pederson, A. Tiselius, Ultracentrifugal and electrophoretic studies on antibodies. Nature 138, (1936).
- W. Boyd, H. Bernard, Quantitative changes in antibodies and globulin fractions in sera of rabbits injected with several antigens. J Immunol 33, 111-122.
- S. Bayne-Jones, Equilibria in precipitin reactions: the coexistence of a single free antigen and its antibody in the same serum. J Exp Med 25, 837-853 (1917).
- S. Hooker, W. Boyd, The existence of antigenic determinants of diverse specificity in a single protein. III. Further notes on crystalline hen and duck-ovalbumins. J immunol 30, 41-49 (1936).
- C. Kanagaratham, B. Sallis, E. Fiebiger, Experimental Models for Studying Food Allergy. Cell Mol Gastroenterol Hepatol 6, 356-369.e351 (2018).
- A. Nials, S. Uddin, Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech 1, 213-220 (2008).
- B. Martel, P. Lovato, W. Bäumer, T. Olivry, Translational Animal Models of Atopic Dermatitis for Preclinical Studies. Yale J Biol Med 90, 389-402 (2017).
- C. Korupalli, et al., Single-injecting, bioinspired nanocomposite hydrogel that can recruit host immune cells in situ to elicit potent and long-lasting humoral immune responses. Biomaterials 216, 119268 (2019).
- Y. Dölen, et al., Nanovaccine administration route is critical to obtain pertinent iNKt cell help for robust anti-tumor T and B cell responses. OncoImmunology 9, 1738813 (2020).
- C. Cekic, Y. Day, D. Sag, J. Linden, Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 74, 7250-7259 (2014).