Over the past few decades, significant advancements have been achieved in recombinant collagen research, particularly in the areas of genetic engineering, protein expression, and biomaterial fabrication. Recombinant human collagen has gathered increasing attention as a promising biomaterial for tissue engineering and regenerative medicine applications.
To produce recombinant collagen, various expression systems—including mammalian, insect, yeast, and bacterial cells—have been developed. Among these, mammalian systems such as Chinese hamster ovary cells are preferred because their translational and post-translational machinery closely resemble those of human cells, allowing proper hydroxylation and glycosylation of collagen molecules.
Verification of recombinant type I (or type III) collagen expression, distinct from host-derived collagen, is essential for product validation and quality assurance. Chondrex, Inc. offers monoclonal antibodies (mAbs) specific to human type I and type III collagens, suitable for use in immunoblotting (IB) and immunoprecipitation (IP) assays. Because most ELISA kits detect the triple-helical structure composed of two α1and one α2 chains in type I collagen, they often exhibit reduced sensitivity. Therefore, non-ELISA methods such as IB and IP are generally more reliable for identifying recombinant collagen.
Here, Chondrex, Inc. further confirmed the specificity of anti-human type I collagen mAbs, demonstrating their precise recognition of collagen chains constituting type I collagen. This specificity is critical for ensuring accurate results in immunoblotting analyses.
Table 1. Monoclonal Antibodies Reacting to Human Type I Collagen
Catalog# | Antibody Name | Specificity | Subtype/Subclass | ||
Collagen Types | Chains | Species | |||
222b | I | α1, β1 | H, P, B, Ms | Rat IgM | |
1C1F8 | I | α2, β2 | H | Rat IgG2a | |
42r | I | α2, β2 | H | Rat IgM | |
3E9B4 | I and III* | γ | H | Mouse IgG2c | |
2H11B10 | I and III* | α1, β1, β2, γ | H, P | Mouse IgG2c | |
Abbreviations: B: Bovine, H: Human, Ms: Mouse, P: Porcine
*NOTE: Both monoclonal antibodies should recognize an epitope that is conserved between type I and III collagen, and which exhibits 63% amino acid sequence similarity.
Electrophoresis and Immunoblotting analysis
Human type I collagen (ELISA Grade, Cat # 1005) was separated in a 6 % SDS gel under non-reducing conditions. To assess protein separation, the gel was stained with Coomassie Brilliant Blue R-250 (CBB). The separated proteins were transferred to a nitrocellulose membrane. The membrane was then blocked with 0.05M Tris-buffered saline buffer, pH 8.0 containing 2 % Casein and 0.05 % Tween 20 (Casein/TBS) and incubated with 5-10 μg/ml of diluted anti-collagen monoclonal antibodies (mAbs) at 4°C overnight. After washing the membrane with PBS containing 0.05 % Tween 20 (PBST), it was incubated with HRP-labeled goat antibodies against the primary antibody in Casein/TBS at room temperature for 2 hours. After washing the membrane with PBST, the protein bands were visualized by adding TMB for immunoblotting.
Results
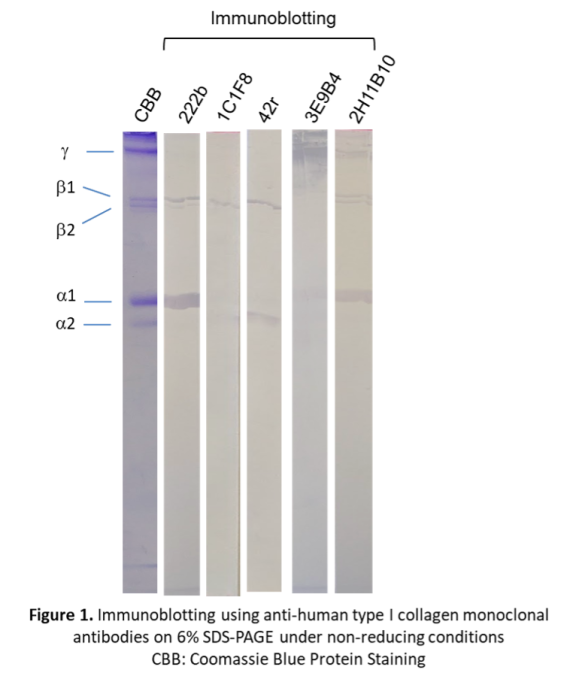
CBB protein staining of human type I collagen revealed the characteristic α1 and α2 monomeric chains, along with β1(α1x2), β2(α1+α2) dimeric chains, and γ (α1x2+α2) trimeric chains. MAbs 222b and 2H11B10 specifically recognized the α1 chain; additionally, 222b bound the β1 chain, whereas 2H11B10 reacted with β1, β2, and γ chains. In contrast, mAbs 1C1F8 and 42r recognized the α2 and β2 chains. Notably, mAb 3E9B4 exhibited exclusive reactivity toward the γ chain.