Type II collagen derived from chicken cartilage has been extensively characterized and widely documented in scientific literature. Recently, type II collagen isolated from salmon cartilage has emerged as a promising source of type II collagen (1). However, before this material can be confidently adopted for analytical or research applications, its biochemical purity and compatibility with established laboratory methods must be thoroughly validated.
In this study, Chondrex Inc. evaluated purified salmon type II collagen (SII) using a native type II collagen ELISA kit (Cat # 6018). The objectives were twofold: (1) to verify the purity and molecular identity of SII via SDS–PAGE and Western blot (WB) analyses, and (2) to determine its reactivity profile within the ELISA assay, which uses chicken type II collagen (CII) as the standard. Accurate quantification of SII requires establishing a cross-reactivity factor and assessing assay linearity to ensure consistent and precise measurement. The results presented here define key validation parameters for the effective use of salmon type II collagen with the Type II Collagen ELISA kit.
1. Purified salmon type II collagen analysis
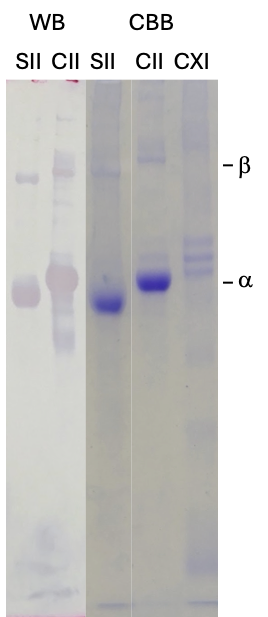
Figure 1. Immunoblotting on 6% SDS-PAGE under reducing conditions using salmon and chick type II and type XI collagen with anti-type II collagen monoclonal antibodies.
SII: Salmon type II collagen, CII: Chick type II collagen, CXI: Chick type XI collagen, CBB: Coomassie Brilliant Blue staining, WB: Immunoblotting
6% SDS-PAGE analysis demonstrated that the α-chain bands of SII migrated to a lower molecular weight compared to those of CII (Figure 1). The purity of the SII was estimated to be greater than 95% by a densitometry analysis. Furthermore, the SII fraction contained minimal or undetectable amounts of type XI collagen when compared with chick type XI collagen. WB analysis using anti–type II collagen antibodies (Cat # 7006) successfully identified both the α-chain and β-chain bands for both SII and CII, thereby validating the presence of type II collagen.
2. Native type II collagen ELISA (Cat # 6018) using chicken type II collagen standards and salmon type II collagen samples.
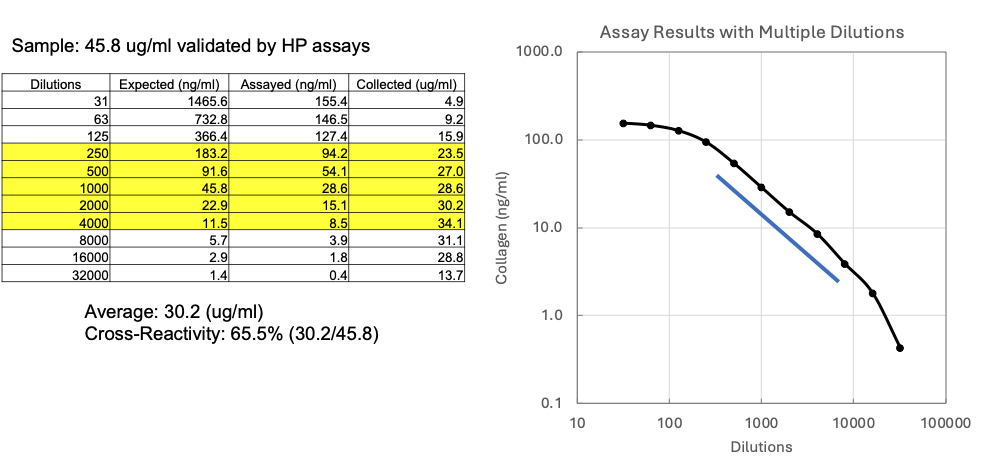
Figure 2. Sample cross-reactivity analysis by ELISA kit using samples validated by hydroxyproline analysis
First, the absolute concentration of the purified SII sample was determined using a hydroxyproline (HP) assay, which is considered the gold standard for collagen analysis. The HP assay confirmed a concentration of 45.8 μg/ml for the SII sample solution.
Next, a series of dilutions, starting at 1:31, were prepared from the sample solution and analyzed using the native Type II collagen ELISA Kit (Cat # 6018). The ELISA response for SII showed signal saturation at concentrations above 100 ng/ml of the kit's chicken type II collagen standard. Therefore, to ensure accurate quantification within the assay's linear range, only values below 100 ng/ml were used for calculation (Figure 2).
Specifically, concentrations of 54.1 ng/ml and 8.5 ng/ml (which correspond to sample dilutions 1:500 and 1:4000, respectively) were selected because they were within the assay's linear range (blue line). Based on these results, the calculated concentration of the SII sample was 30.2 μg/ml.
Finally, assuming the purified SII was composed entirely of native collagen, the cross-reactivity of SII in this ELISA kit was calculated by comparing the ELISA-derived concentration (30.2 μg/ml) with the HP-validated concentration (45.8 μg/ml). The resulting cross-reactivity was 65.5% (30.2/45.8).
3. Linearity of salmon type II collagen in native type II collagen ELISA (Cat # 6018)
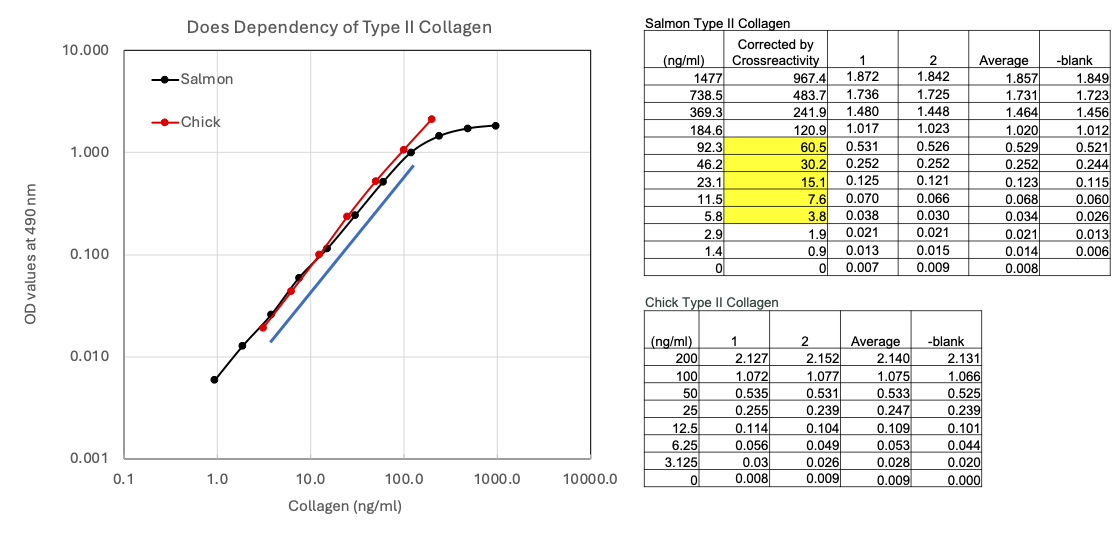
Figure 3. Comparing the linearity of salmon and chick type II collagen in ELISA
The linearity of the SII samples corrected with 65.5% cross-reactively in the ELISA was confirmed to be less than 100 ng/ml of the kit's chicken type II collagen standard (Figure 3). While the chicken standard exhibited good linearity up to 200 ng/ml, the SII samples demonstrated a saturation effect above the 100 ng/ml chick standard concentration. This saturation is currently under investigation, but potential causes for this effect include tertiary structure inhibition impacting the monoclonal antibody epitopes, or a limited availability of epitopes on the SII triple helix structure, possibly due to differences in amino acid sequence homology between salmon and chicken type II collagen. Nevertheless, assay results below 100 ng/ml remain suitable for accurate analysis.
4. Conclusion
In conclusion, although a saturation effect was observed at higher concentrations, salmon type II collagen could still be reliably quantified using the Cat # 6018 ELISA kit by limiting the analysis to concentrations below 100 ng/ml of the kit's chicken type II collagen standard curve. To obtain accurate results, all assay results should be converted to absolute salmon type II collagen concentrations using a conversion factor of 100/65.5, which accounts for the observed cross-reactivity.
Reference