Collagen is the predominant structural protein in the extracellular matrix (ECM) of animals. Due to its unique structure and complex tissue distribution, analyzing collagen from tissue and cell culture samples presents many unique challenges. Here, Chondrex, Inc. provides an overview of collagen structure, immunostaining patterns, and information on type I and type II collagen preparations.
1. Type I and Type II Collagen Structure
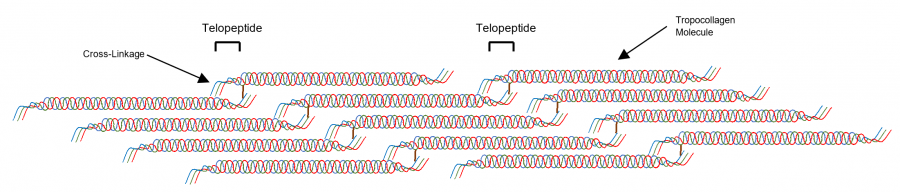
Fibrillar collagen consists of three alpha (α)-chains (type I collagen: two α1-chain and one α2-chain and type II collagen: three α1-chains) that self-assemble into a left-handed triple helix structure. Individual collagen triple helixes are termed tropocollagen. The triple helix chains are stabilized through cross-linkages at the telopeptide regions of adjacent collagen helixes (Figure 1). These cross-linked tropocollagen molecules comprise collagen fibrils.
Figure 1. Structure of a collagen fibril. Tropocollagen molecules are cross-linked by bonds at their telopeptide sequences, forming collagen fibrils. Collagen fibrils provide structural support in the ECM. The cross-linkages between telopeptide sequences of α-chains increase the strength of collagen fibers.
In acidic conditions pepsin, an acid proteinase, cleaves the terminal telopeptide sequence to produce atelocollagen (Figure 2). This process preserves the triple helix structure and facilitates solubilization of atelocollagen. Pepsin digestion of collagen allows for the removal of the telopeptide portion of collagen, which is highly immunogenic (antigenic). The atelocollagen, generally referred to as “collagen” by researchers, has been used extensively for preparing industrial materials and biological research, especially for human use.
Atelocollagen becomes denatured and loses the triple-helix structure at temperatures higher than 42°C, degrading the collagen molecules into its three individual α-chains (Figure 2). Interestingly, a stepwise temperature cooling procedure can partially reassemble the denatured α-chains into the triple helix structure; a process known as renaturation. Unfortunately, the internal cross-linkages between α-chains (especially the high degree of cross-linking seen in in collagen from older tissues) cannot be reformed through the renaturation process, preventing the reforming of native collagen in tissues. Therefore, denaturation of collagen is irreversible. Additionally, collagen is highly resistant to most proteinase or peptidase activity. However, once collagen is denatured, it becomes highly susceptible to many proteinases and will be easily digested into small peptides.
Figure 2. Pepsin digestion will cleave the telopeptide sections of the tropocollagen molecules, resulting in atelocollagen. When atelocollagen is exposed to temperatures above 42°C, it loses its triple-helix structure, resulting in three individual α-chains.
2. Analyzing Collagen Preparations: SDS-Gels, Western Blots, and ELISAs
In aging tissues, collagen will obtain intra- and inter-collagen cross-linkages between lysine residues in α-chains due to oxidation. The intra-collagen cross linkages can result in two specific structures: a beta (β)-chain (cross-links between two α-chains), and a gamma (γ)-chain (cross-links between three α-chains) (Figure 3). Inter-collagen cross-linkages (links between collagen fibers) create polymeric collagen (Figure 4).
Figure 3 (left). Collagen structures generated by intra-collagen cross-linkages.
Figure 4 (right). Polymeric collagen generated by inter-collagen cross-linkages.
SDS-gel analysis can be used to analyze and evaluate collagen qualities and production. Atelocollagen has a molecular weight (MW) of 300 kDa. Gel-analysis requires denaturation of samples by heating with SDS to unfold and linearize the protein structures. A 6% gel-analysis of collagen shows a single α-chain with a MW of 100 kDa, as well as β-chains (200 kDa) and γ-chains (300 kDa) (Figure 5). Furthermore, type I collagen will show two different α-chains, two different β-chains and a single γ-chain (five bands total) due to the different combinations of α-chains that comprise type I collagen fibers (Figure 5). The ratio of the α-, β-, and γ-chains in a collagen gel analysis will depend on the degree of cross-linkages in the sample. Polymeric collagen, which contains both intra- and inter-collagen cross-linkages, will show bands higher than MW 300kDa in the SDS-page analysis.

Figure 5. Collagen analysis by 6% SDS-Gel under non-reducing condition with Coomassie Brilliant Blue (CBB) staining and Western Blot (WB) using A) chick type II collagen and B) rat type I collagen. 2 ug collagen was loaded on a 6% gel under non-reducing condition and run at 100V for 2 hours. The collagen was transferred to a nitrocellose membrane at 25V for 2 hours. WB was proceeded with a primary antibody (10 ug/ml) at 4C overnight incubation, and anti-mouse IgG conjugated with peroxidase at room temperature for 2 hours. Antibody binding was visualized by 3,3-diaminobenzidine.
Collagen can be detected by western blot (WB) analysis using anti-collagen antibodies following gel-analysis (Figure 5). Because anti-collagen antibodies are generally specific to native collagen, a higher concentration of primary antibodies is required for WB analysis. Additionally, the epitope of antibodies, especially monoclonal antibodies, will influence the immunostaining patterns. For example, anti-type II collagen monoclonal antibodies (Cat #7005), react to α-chains, showing all three (α, β, and γ) bands in WB (Figure 5 A), but an anti-rat type I collagen monoclonal antibody (Cat #7043) showed only 1 β-band (Figure 5 B). Selecting the proper anti-collagen antibody is vital for WB analysis of collagen samples. Please contact us for assistance with choosing the best antibody for your study.
Type I and type II collagen levels from tissue and cell culture samples can be quantified using Chondrex, Inc.'s Collagen Detection ELISA Kits. It is important to note that the type I and type II collagen detection kits, as well as the Sirius Red Total Collagen Detection Kit, are highly specific for native form collagen (atelocollagen) and have poor reactivity with denatured collagen. If using these kits, please contact us to receive our sample preparation protocols for these kits.
3. Collagen Solubilization and Preparation
Chondrex, Inc. provides highly purified collagen of several different grades, each suitable for different study purposes. Most of our collagen is pepsin solubilized collagen (atelocollagen) which is fractioned by repeatedly alternating salt precipitation under acidic conditions and neutral pH conditions.
Individual collagen types have unique solubilities depending on the salt concentration and buffer pH (Table 1). In an acidic buffer, type I, II and III collagens are precipitated at 0.7M NaCl; type IV and V collagen are precipitated at 1.2M NaCl. On the other hand, in a neutral pH buffer, type III and IV collagen are precipitated at 1.8M NaCl, type I collagen at 2.5M NaCl and type II collagen at salt concentrations higher than 4.0M NaCl.
Table 1. Different NaCl concentrations can precipitate different types of collagen, depending on buffer pH (1).
Ion-exchange chromatography is used to remove contaminated pepsin during the collagen solubilization step. Pepsin is a strong antigen to T-cells, therefore removing pepsin is required for studying the pathogenesis of collagen in immune functions. Furthermore, collagen is insoluble at low concentrations of phosphate buffer (0.02M) without NaCl. The precipitation of collagen in phosphate buffer works to remove water soluble contaminants.
For over 20 years, Chondrex, Inc. has prepared highly purified collagen using these purification techniques. Our scientists are available to provide assistance and advice for in vitro and in vivo studies using collagen. We routinely validate our Immunization Grade Type II Collagen from bovine and chicken sources for use in the Collagen-Induced Arthritis (CIA) Model in DBA/1 mice. If you have any questions concerning collagen preparation, collagen analysis, or the CIA model, please contact us.