Matrix metalloproteinases (MMPs, aka matrixins) are zinc-dependent endopeptidases that are members of the metzincin-superfamily of enzymes. The first MMP (MMP-1) was discovered in tadpoles during embryogenesis (1), hinting at the prominent role that MMPs play in growth and development. Indeed, MMPs are a collection of enzymes that are chiefly responsible for the physiological turnover of extracellular matrix (ECM) and connective tissues in embryogenesis, organogenesis, angiogenesis, and tissue repair/remodeling. While typically found in low amounts in healthy adult tissues, MMP activity can be upregulated in pathologies where turnover of connective tissues and ECM are a defining feature, such as: arthritis (2), chronic allergic asthma (3), cancer (4), nephritis (5), and fibrotic diseases (6).
All MMPs share two domains: a propeptide domain and an N-terminal catalytic domain containing a Zn2+ ion critical for MMP proteinase activity. Most MMPs also possess a C-terminal hemopexin-like domain that is vital for binding to collagen fibers, although MMP-7, MMP-23, and MMP-26 lack this domain. MMPs released into the extracellular space are typically zymogens, latent enzymes that need to be activated by other enzyme activity. A cysteine residue in the propeptide domain blocks the active site by interacting with the Zn2+ at the active site, rendering the enzyme inactive. Chemical modification or proteolytic removal of the pro-peptide domain by other proteinases, including other MMPs, will activate the MMP molecule. This activation mechanism, called a cysteine switch, is common to almost all MMP molecules identified thus far (7).
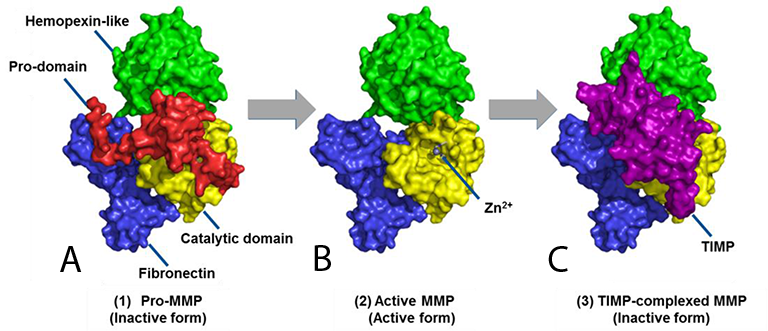
Figure 1. Typical tertiary structure of soluble MMP showing the A) Inactive pro-MMP (zymogen), containing the pro-domain, B) Active MMP (with the pro-domain enzymatically removed and the Zn2+ ion in the active site exposed), and C) andTIMP complexed with MMP (inactive).
Additionally, MMPs have been categorized based on their substrate specificities and their form. Membrane-type MMPs (MT-MMP) are incorporated into cell membranes by a transmembrane domain or tethered to cell membranes by a glycosylphosphatidylinositol (GPI)-anchor. Soluble MMPs (collagenases, gelatinases, stromelysins, matrilysins, macrophage elastase) can be found free in the ECM or in cell vesicles where they are stored until stimulated for release. These soluble MMP classifications are based on shared substrate specificities and similarities in their structures:
- Collagenases (MMP-1, MMP-8, MMP-13) are MMPs with the ability to cleave interstitial collagen (native form, fibrillar collagen; type I, II, III). This collagenase cleavage results in an N-terminal Ύ length peptide and a C-terminal Ό length peptide.
- Gelatinases (MMP-2, MMP-9) primarily degrade denatured collagen, collagen fragments (like those generated by collagenase cleavage), and non-fibrillar collagen (types IV, V).
- Stromelysins (MMP-3, MMP-10, MMP-11) and matrilysins (MMP-7, MMP-26) exhibit a much wider substrate specificity, degrading many different ECM components: proteoglycans, fibronectin, laminin, gelatin and others.
- Macrophage Metalloelastase (MMP-12) primarily degrades elastin, but also many other ECM constituents.
However, MMPs appear to exhibit a much broader proteolytic activity (at least in vitro) than originally thought. Many MMPs have proteolytic activity against numerous ECM proteins, as well as non-ECM components, such as growth factor-binding proteins (8), cell surface growth factor receptors (8), as well as cytokines and chemokines (9). This ability to modulate inflammatory signaling and chemotactic signaling molecules intimates a wider regulatory role for MMPs than initially thought. However, proteolytic processing of cytokines and chemokines by MMP is a complicated pathway. Indeed, cleavage of cytokines and chemokines by various MMPS can lead to activation or inactivation of the signaling molecule, with either agonist or antagonist effector functions (9). More research is needed to determine the how MMP cleavage of cytokines and chemokines affects immune responses and disease progression.
The table below provides more information on the reported substrates of most MMPs, as well as the physiological functions associated with MMP activity.
| Also Known As | Category | Substrates | Physiological Functions | |
|---|---|---|---|---|
| MMP-1 | Collagenase 1 Interstitial Collagenase Fibroblast Collagenase | Collagenase | Aggrecan, Versican, Perlecan, Nidogen, Serpins, Tenascin C, Native Collagen, CXCL12, CCL7, CCL2, CCL13 | Embryogenesis |
| MMP-2 | Gelatinase A 72-kDa Gelatinase Type IV Collagenase Neutrophil Gelatinase | Gelatinase | Gelatin, Collagen (I, IV, V), Elastin, Vitronectin, IL-1β, TGF-β, CXCL12, CX3CL1, CCL7 | Neovascularization/ Angiogenesis, Inflammatory signaling regulation; Ectodomain shedding |
| MMP-3 | Stromelysin 1 Transin 1 | Stromelysins | Fibronectin, Gelatin, Laminin, Proteoglycans, Globular Type IV, IL-1β, TGF-β, CXCL12, CCL2, CCL7, CCL8, CCL13 | MMP activation; Wound healing; Involution |
| MMP-7 | Matrilysin 1 PUMP-1 protease Uterine Metalloproteinase | Matrilysin | Fibronectin, Gelatin, Laminin, Elastin, proMMP-2, proMMP-9 | Endometrial involution; Mucosal immunity; Wound repair |
| MMP-8 | Collagenase 2 Neutrophil Collagenase | Collagenase | Collagen (I, II, III, V, VII, VIII, X), Gelatin, Aggrecan, Fibronectin, CXCL5, CXCL8, CXCL9, CCL2, CXCL5 | Embryogenesis; Uterine tissue remodeling |
| MMP-9 | Gelatinase B 92-kDa type IV Collagenase | Gelatinase | Collagen (IV), IL-8, IL-1β, TGF-β, IFN-γ CXCL12, CXCL4, CXCL1, CTAPIII, CXCL5, CXCL8, CXCL9 | Neovascularization; Immune cell migration; Endometrial tissue remodeling; Embryo implantation |
| MMP-10 | Stromelysin 2 | Stromelysins | Collagen (III, IV, V), Gelatin, Aggrecan | Bone Remodeling, Ossification; Wound healing; Cell migration |
| MMP-11 | Stromelysin 3 | Stromelysins | Casein, Fibronectin, Vitronectin, Laminin, Entactin, Proteoglycans, Fibrinogen, Fibrin, Plasminogen | -- |
| MMP-12 | Macrophage Elastase | Elastase | Elastin, Plasminogen | -- |
| MMP-13 | Collagenase 3 | Collagenase | Collagen (I, II, III, IV, IX, XIV), Gelatin, Aggrecan, Perlecan, Fibronectin, Tenascin-C, CXCL12, CCL7 | Bone Development; Angiogenesis; |
| MMP-14 | MT1-MMP | Membrane-Type | Collagen (I, II, III), Fibronectin, Vitronectin, Tenascin, Nidogen, Aggrecan, Fibrin, Fibrinogen, Laminin-5, proMMP-2, proMMP-13, proMMP-8, TGF-β, CXCL12, CCL7 | Angiogenesis; Endothelial cell migration |
| MMP-15 | MT2-MMP | Membrane-Type | Collagen (I, II, III), Fibronectin, Vitronectin, Tenascin, Nidogen, Aggrecan, Fibrin, Fibrinogen, Laminin-5, proMMP-2, proMMP-13, proMMP-9 | Ovulation (follicle rupture) |
| MMP-16 | MT3-MMP | Membrane-Type | proMMP-2 | -- |
| MMP-17 | MT4-MMP | Membrane-Type | Gelatin, Fibrin, Fibrinogen | -- |
| MMP-20 | Enamelysin | N/A | Amelogenin | Tooth enamel turnover |
| MMP-25 | MT6-MMP Leukolysin | Membrane-Type | Gelatin, Fibrin, Fibrinogen, Fibronectin, Collagen (IV), Proteoglycans, proMMP-2 | -- |
| MMP-26 | Matrilysin 2 Endometase | N/A | Fibronectin, proMMP-2, proMMP-9 | -- |